-
Environmental catalysis on iron oxide-silica aerogels: Selective oxidation of NH3 and reduction of NO by NH3
P. Fabrizioli, T. Bürgi and A. Baiker
Journal of Catalysis, 206 (1) (2002), p143-154


DOI:10.1006/jcat.2001.3475 | unige:14696 | Article HTML | Article PDF
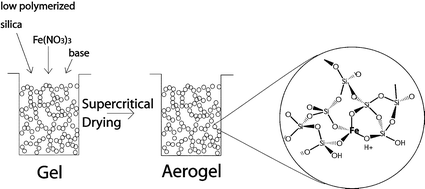
The catalytic properties of mesoporous iron oxide–silica aerogels prepared by a sol–gel process combined with ensuing supercritical extraction with CO2 was investigated in the selective oxidation (SCO) of ammonia and the selective reduction (SCR) of NO by ammonia. The main parameters changed in the aerogel preparation were the type of base used as gelation agent, the iron content, and the calcination temperature. The aerogels differed significantly in acidity and iron dispersion. Diffuse reflectance infrared Fourier transform spectroscopy studies of ammonia adsorption at different temperatures revealed that ammonia was bound to Brønsted- and Lewis-type sites, the latter being dominant at 300°C. A fraction of low coordinated Fe2+ sites were probed by NO adsorption measurements. Lewis-type sites were found to be associated with low-coordinated iron sites. Catalytic tests were performed in a continuous fixed-bed reactor in the temperature 210–550°C range and at ambient pressure. The catalytic activity of the aerogels in SCO correlated with the abundance of more strongly bound ammonia adsorbed on Lewis sites (low coordinated iron). High selectivity to nitrogen (97%) could be reached up to 500°C, whereas at higher temperature the formation of N2O and NO became significant. The apparent activation energy of N2 formation ranged from 69 to 94 kJ/mol, whereby catalysts with higher selectivity and activity showed lower activation energy. In SCR, selectivity to nitrogen was for all aerogels >98% at T<460°C, and activation energies varied from 38 to 53 kJ/mol. The catalytic activity for SCR did not correlate with the population density of Lewis sites. We propose that SCO predominantly occurs on Lewis sites consisting of highly dispersed iron atoms of low coordination, whereas in SCR these sites do not play an important role.